
Why measure it?
In the upper atmosphere ‘good’ ozone (O3) protects life on Earth from the sun’s ultraviolet rays. At ground level ‘bad’ ozone is a criteria pollutant that is a significant health risk, especially for people with asthma. It also damages crops, trees and other vegetation and is a main component of smog.
Where does it come from?
Natural Sources
Most ozone (about 90%) resides in a layer that begins between 6 and 10 miles (10 and 17 kilometers) above the Earth’s surface and extends up to 30 miles (50 kilometers). This region of the atmosphere is called the stratosphere. The O3 in this region is commonly known as the ozone layer. Atmospheric turbulence and mixing of this layer into the lower troposphere results in a natural background concentration of about 0.03 to 0.04 ppm (30 to 40 ppb) of O3 at ground level.
As a Pollutant
Ground level ozone above the natural background is not emitted directly but is created by chemical reactions between the precursors; oxides of nitrogen (NOx) and volatile organic compounds (VOC) in the presence of sunlight. The major sources of NOx and VOC are industrial facilities, vehicle exhaust, gasoline vapors and chemical solvents. The O3 reaction dynamics are such that concentrations are often highest downwind of the precursor sources and on the outskirts of urban areas.
Industrial Uses
Ozone has found a range of industrial uses primarily as a disinfectant or sterilizing agent. It is used extensively in the food and beverage industry, water treatment, manufacturing, odor control, and sterilization in medial and domestic environment.
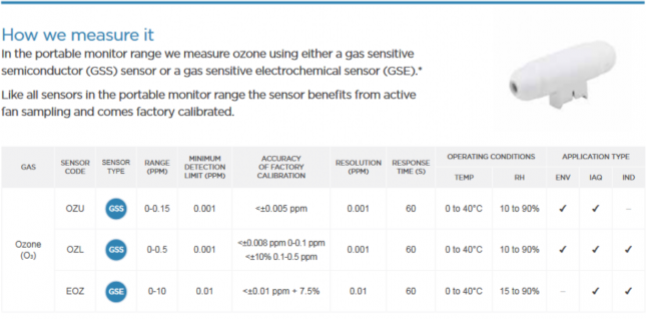
How we measure it
More information on the Aeroqual Gas Detection Monitors can be found on Gas Sensing website, using the links below.

