Chloride Information

Chloride:
|
Other Names |
Chloride ion |
|
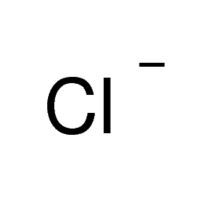
Chemical Formula |
Cl- |
|
CAS Number |
16887 – 00 – 6 |
|
Industry Uses |
Salts; Chemical Intermediate; Water Treatment |
|
Health Risks |
Skin reaction |
What is Chloride:
Chloride is a chlorine anion that forms the negatively charged part of many salts. It is the most common inorganic anion in water and wastewater. Natural sources of salt are the ocean and salt deposits above and below ground. It is an essential electrolyte in all body fluids for maintaining acid/base balance, transmitting nerve impulses, and regulating fluid flow in and out of cells. Chloride is very corrosive to most metals in systems with elevated pressures and temperatures such as boilers and oil-drilling equipment. Chloride plays an important role in desalination, which removes chloride salts to make potable water.
Chloride Exposure and Health Risks:
Direct contact can cause an allergic skin reaction. Health risks depend on the other ion present with chloride. Sodium chloride, known as table salt, is harmless. Calcium, magnesium, and potassium chlorides have varied uses ranging from medical treatments to de-icing to cement formation. Hydrogen chloride can be extremely dangerous and even toxic.
Regulations:
The table below summarizes the most-recent standards.
|
Limit/Level |
Type |
Organization |
|
250 mg/L |
Secondary Standards – Max Contaminant Level |
EPA |
Sources: EPA
Measuring Chloride:
Dissolved chloride concentration in water can be measured in units of parts per million (ppm). We carry kits from CHEMetrics that determine chloride levels in water in ranges from 20-200 ppm to 10,000-100,000 ppm. All of our chloride products can be viewed HERE.
What type of component are you looking for?
| Fixed Mount | Handheld | Dissolved Kits: | Replacement Sensors: | Calibration Gas: | Rentals: |
 |
 |
 |
 |
 |
 |
All sensors require a yearly calibration to ensure your gas measurements are accurate and performing within manufacturer standards.
**Calibration Service Request Form **
|
Calibration costs do vary, see below to get an estimate: Calibration Fee: $150 Analyzer Calibration Fee: $300 PM Calibration Sensor Fee: $330 Genie Calibration Fee: $265 ATI Calibration Fee: $205 ** note that prices are subject to change per labor and parts required. |
Contact us for help choosing the right product for your application