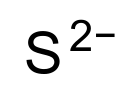Sulfide Information

Sulfide:
|
Other Names |
Sulphide; Sulfide ion; Sulfanediide |
|
Chemical Formula |
S-2 |
|
CAS Number |
18496 – 25 – 8 |
|
Industry Uses |
Metallurgy; Chemical Manufacture; Chemical Laboratories; Fertilizers |
|
Health Risks |
Reacts to form Toxic Gas |
What is Sulfide:
Sulfide is an inorganic anion of sulfur. It is a strong base and good reducing agent. Sulfides are naturally present in ground waters as a result of leaching from sulfur-containing mineral deposits. Surface waters usually do not contain high sulfide concentrations. Sulfides result from the decomposition of organic matter, bacterial sulfate reduction under anaerobic conditions, and various chemical processes. Dissolved free sulfides are very aggressive corrosives especially to metals like steel, stainless steel, and copper. Sulfides in aqueous solution are responsible for stress corrosion cracking of steel.
Sulfide Exposure and Health Risks:
Sulfides readily form Hydrogen Sulfide which is a very toxic and corrosive gas. Some sulfides are highly flammable, and when they burn they produce sulfur dioxide gas.
Regulations:
The EPA and CDC do not have any listed restrictions or regulations on sulfide levels
Measuring Sulfide:
Dissolved Sulfide concentration in water can be measured in units of parts per million (ppm). We carry instrumental kits from CHEMetrics and monitors from ATI that determine Sulfide levels in water. All of our Sulfide products can be viewed HERE.
What type of component are you looking for?
| Fixed Mount | Handheld | Dissolved Kits: | Replacement Sensors: | Calibration Gas: | Rentals: |
 |
 |
 |
 |
 |
 |
All sensors require a yearly calibration to ensure your gas measurements are accurate and performing within manufacturer standards. This page is desiccated to the individual manufacturers we represent and their specific calibration procedures.
**Calibration Service Request Form **
|
Calibration costs do vary, see below to get an estimate: Calibration Fee: $150 Analyzer Calibration Fee: $300 PM Calibration Sensor Fee: $330 Genie Calibration Fee: $265 ATI Calibration Fee: $205 ** note that prices are subject to change per labor and parts required. |
Contact us for help choosing the right product for your application