Ammonia is a compound that might not be a household name, but its presence is felt across various industries and applications. From fertilizers to pharmaceuticals, cooling systems to cleaning agents, ammonia plays a crucial role in many processes. However, the benefits come with risks, and understanding the properties, uses, and health hazards of ammonia is essential for ensuring safety in its handling and use.
What is Ammonia?
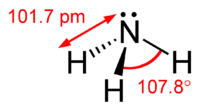
Ammonia, with the chemical formula NH3, is a nitrogen-based compound. While it is colorless, it possesses a distinctive, pungent smell even at low levels. You might have encountered this scent in household cleaners, smelling salts, or agricultural settings. Ammonia is a natural byproduct of decomposing organic matter, animal waste, and it even exists in our solar system.
Despite its ubiquity in nature and industrial applications, ammonia is classified as an extremely hazardous substance. This is primarily due to its caustic and flammable nature, making it crucial to understand its properties and safety precautions.
Properties of Ammonia
- CAS Number: 7664-41-7
- Industry Uses: Fertilizers, Cleaners, Cooling Systems, Pharmaceuticals
- Health Risks: Irritation to eyes, airways, sinuses, and throat; neurotoxin and metabotoxin; burns and coughing
- Vapor Pressure: 857.3 kPa
- Water Solubility: Soluble
- Flammability: Flammable
- Odor: Pungent; familiar to many as it is used in smelling salts and household cleaners
Ammonia Exposures and Health Risks
Ammonia can be found in many household cleaners, agricultural fertilizers, cooling and refrigeration systems, and pharmaceuticals. Despite its widespread use, even brief exposure to small concentrations of ammonia can be extremely dangerous. It is toxic whether inhaled or ingested, posing a threat to aquatic life as well.
Ammonia is also an irritant, particularly to the eyes, nose, throat, and lungs. Contact with the eyes can result in blindness, and burns and coughing are common side effects of ammonia exposure. Notably, ammonia is both a neurotoxin, causing damage to neural tissue and cells, and a metabotoxin, leading to various adverse health effects.
Regulations
To safeguard against ammonia exposure, regulatory agencies have set exposure limits. Here are some of the most recent exposure limits:
- 30 ppm (AEGL-1, 8 hrs) – EPA
- 110 ppm (AEGL-2, 8 hrs) – EPA
- 2700 ppm (AEGL-3, 10 min) – EPA
- 390 ppm (AEGL-3, 8 hrs) – EPA
- 25 ppm (TWA, 10 hrs) – NIOSH
- 35 ppm (Short Term Exposure Limit) – NIOSH
- 50 ppm (PEL, 8 hrs) – OSHA
Measuring Ammonia
To ensure safety, it is essential to measure ammonia accurately, whether in air or liquid form. Commonly, ammonia is measured in parts per million (ppm) for human safety purposes and in percent by weight for commercial production and storage applications. The most prevalent method of measurement is through Electrochemical Gas Sensors, which are used in various ammonia products.
Our ammonia monitors, available in portable and fixed configurations, are designed to meet your monitoring needs. We also offer liquid (dissolved) ammonia sensors and replacement sensors.
All our Ammonia Monitors can be found here: https://www.gas-sensing.com/support/gas-information/ammonia-sensors.html
Calibration Services
Maintaining the accuracy of your ammonia sensors is critical for safety. Regular calibration ensures that your gas measurements align with manufacturer standards. Different manufacturers have specific calibration procedures, and the costs can vary. To request calibration services, you can use the provided form on our website.
Contact Us for Expert Guidance
If you’re unsure about the right ammonia sensor for your application or need assistance with any aspect related to ammonia safety, don’t hesitate to contact us. Your safety is our priority, and we’re here to help you make informed decisions and keep your operations safe.
Stay informed, stay safe, and make responsible choices when dealing with ammonia, an essential yet potentially hazardous compound in various industrial and household settings.