Ammonia (NH3) Defined:
Ammonia is a compound made up of two elements, nitrogen and hydrogen (NH3). Throughout human history, it has always been a useful chemical although it was not until recently (the past 100 years) that we have been able to produce ammonia in large quantities. In ancient times and the middle ages, ammonia was used for cleaning, for tanning leather hides, and for dying cloth – but how did they produce their ammonia? Fermented urine. In all mammals’ bodies, kidneys produce ammonia to neutralize acids in the body; as anybody who has changed a baby’s diaper or gotten too close to a rabbit cage knows, that ammonia passes out of the body in urine and contributes to its pungent odor (to be more scientifically accurate, the ammonia is technically converted to urea in the body, and then begins converting back to ammonia after exiting the body – the fermenting process hastens this post-body conversion). In more recent times, two German chemists invented a process that creates ammonia from air. The Haber-Bosch process, named after its inventors, was perfected in the decades between the World Wars, and earned both its inventors the Nobel Prize – people were excited about ammonia production not for cleaning or dying cloth, but for its use in fertilizer. Around the time Haber and Bosch were perfecting their process, experts were predicting mass starvation for the world’s growing population; now, roughly 100 years after the discovery of how to turn air into ammonia, the world’s population has grown by several billion and more people face the problem of obesity than of starvation. In addition to its use in making fertilizer, ammonia today is also used for making explosives and window cleaning solution, and is used for alcohol fermentation (the nitrogen feeds the microorganisms) and disinfecting processed meat (killing off any lurking E. coli).
Reasons to Measure Ammonia (NH3):
Of course, like any chemical, ammonia is good in its proper context, but is harmful in other situations. Because of its low boiling point, ammonia changes from a solid to a gas very quickly, and can damage lungs if breathed in too high a concentration. As a frame of reference, you can start to smell ammonia when it has a concentration of 5 parts per million (ppm), well below the danger threshold. 35 ppm levels for 15 minutes, or 25 ppm levels for 8 hours, is the OSHA (Occupational Safety and Health Administration) permissible exposure limit. The ammonia level identified as IDLH (Immediately Dangerous to Life and Health) is 300 ppm.
Where to Buy the Aeroqual Ammonia Sensor Heads NH and ENG:
The Aeroqual Ammonia Sensor Head (ENG) can be purchased here.
The Aeroqual Ammonia Sensor Head (NH) can be purchased here.
Compatibility with Aeroqual Monitor Bases S200, S300, and S500:
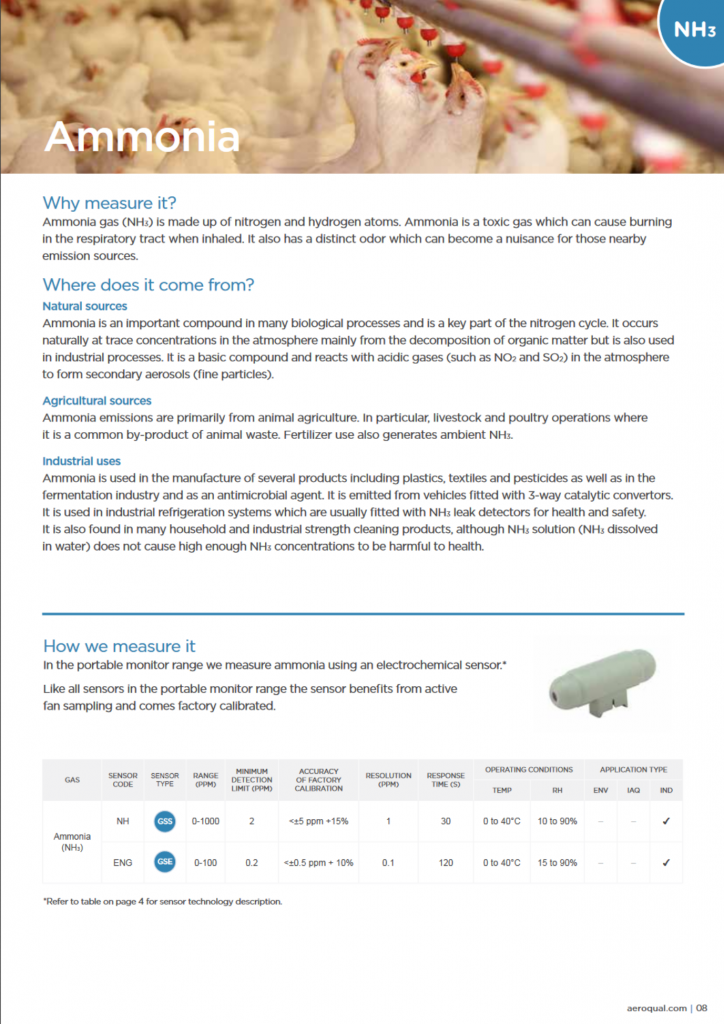
| NH Sensor Features: | NH Sensor Data: |
| Sensor Code: | NH |
| Sensor Technology: | GSS (Gas-Sensitive Semiconductor) |
| Range: | 0-1000 ppm |
| Min Detection Limit | 2 ppm |
| Accuracy: | <+/- 5 ppm +15% |
| Resolution: | 1 ppm |
| Response time: | 30 seconds |
| Temp Operating Range: | 0-40° C (32 – 104° F) |
| RH Operating Range: | 10 – 90% RH |
| ENG Sensor Features: | ENG Sensor Data: |
| Sensor Code: | ENG |
| Sensor Technology: | GSE (Gas-Sensitive Electrochemical) |
| Range: | 0-100 ppm |
| Min Detection Limit | 0.2 ppm |
| Accuracy: | <+/- 5 ppm +10% |
| Resolution: | 0.1 ppm |
| Response time: | 120 seconds |
| Temp Operating Range: | 0-40° C (32 – 104° F) |
| RH Operating Range: | 15 – 90% RH |